Remember that the formula of molarity is the following:
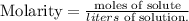
We just have to solve for 'moles of solute' and replace the values that we have of molarity (2.30 M) and volume (0.250 L):
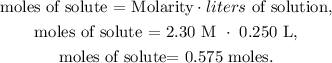
The answer is that we have 0.575 moles of KI in a 0.250 L of a 2.30 M solution.