To solve this problem we have to use ideal gas law.
First convert mL to L, torr to atm and °C to K:
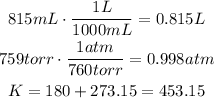
Use the ideal gas law and solve for n:
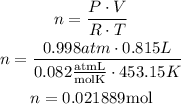
Use Avogadro's number to find the number of molecules in the amount of moles obtained:
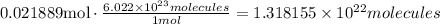
It means that there are 1.318155x10^22 molecules contained in 815mL.