Given,
The latent heat of vaporization of water, L=2.26×10⁶ J/kg
The specific heat capacity of the water, c=4190 J/kg/K
The density of the water, ρ=1000 kg/m³
The volume of the water, V=100 m³
The initial temperature of the water, T₁=18 °C
The final temperature of the water, T₂=100 °C
The mass of the given volume of water is,
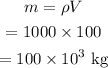
The heat needed to raise the temperature of the water to 100 °C is given by,
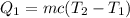
The heat needed to vaporize the water at 100 °C is given by,
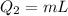
Thus the total heat energy needed is given by,
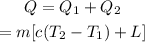
On substituting the known values,
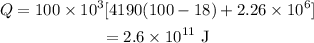
Thus the heat energy needed to evaporate the given amount of water is 2.6×10¹¹ J