We are given that the radioactive decay of a substance is given by the following equation:
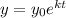
We need to determine the value of "k". To do that we will use the fact that the half-life of the quantity is 60 years. The half-life is the time for the quantity to be half the initial value, therefore, we have:
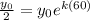
We can cancel out the initial quantity:
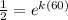
Now, we take the natural logarithm to both sides:
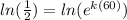
Now, we use the following property of logarithms:
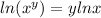
Applying the property we get:
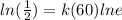
We also have:
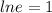
Substituting we get:
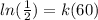
Now, we divide both sides by 60:
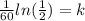
Now, we solve the operations:
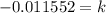
Now, we substitute the value of "k":
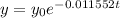
We are given that 100 grams is present today. If today is the value when time "t" is zero then 100 grams is the initial quantity, therefore, we substitute:
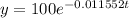
Now, we are asked to determine the time when "y = 68g":
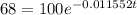
Now, we solve for "t". First, we divide both sides by 100:
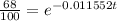
Now, we take the natural logarithm:
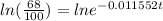
Now, we apply the property of logarithms:
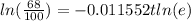
Applying "ln (e) = 1";
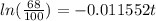
Now, we divide both sides by -0.011552:
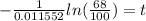
Solving the operation:
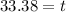
Therefore, the time required is 33.38 years.