1. First, let's state the chemical reaction based on the given description. Copper (Cu) and silver nitrate (AgNO3) are the reactants and there is a single replacement:
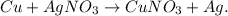
The next step is to convert from grams to moles 15.00 g of Cu, using its molar mass which you can find in the periodic table (63.5 g/mol):
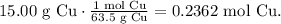
Now, with this value, we can find the number of moles of silver. You can see that in the reaction 1 mol of Cu reacts and produces 1 mol of silver (Ag), so the mol ratio is 1:1, meaning that it is producing the same number of moles of Cu but what would be its mass of 0.2362 moles of Ag? We need to use the molar mass of Ag which is 107.87 g/mol:
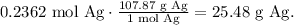
The answer is that 15.00 g of copper (Cu) with an excess of silver nitrate produces 25.48 g of silver (Ag).
2. Let's convert grams of Ag to oz:
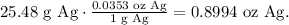
And now, let's see how much would 0.8994 oz of silver we can collect:
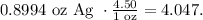
The answer is $4.047.