Step 1
The equations used here:
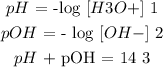
---------------
Step 2
Information provided:
[OH−] = 1.6×10^−5 M (It is the concentration of OH-)
---------------
Step 3
Firstly, we begin with pOH as follows:
pOH = - log [OH−]
pOH = - log (1.6×10^−5 M) = 4.8
---
Secondly, it is needed the equation (3) for pH:
pH + pOH = 14
pH = 14 - 4.8 = 9.2
pH = 9.2
---
Thirdly, From (2), the concentration of H3O+ is found as follows:
pH = - log [H3O+]
It is needed the [H3O+], so it proceeds as follows:
[H3O+] = 10^-pH
[H3O+] = 10^-(9.2) = 6.3x10^-10 M
Answer: [H3O+] = 6.3x10^-10 M