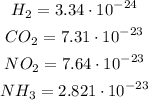Scientific notation
We want to add the indicated atoms mass for each tile
H₂: 2 atoms of hydrogen
Since each hydrogen atom's mass corresponds to
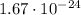
Then H₂:
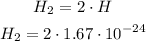
we multiply just the numbers without exponent:
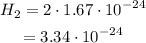
CO₂: 1 atom of carbon and 2 atoms of oxygen
Similarly, to the previous, we know that:
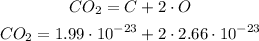
First we multiply the numbers without exponent: 2 * 2.66:
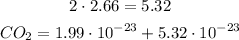
Now, we can add both terms:
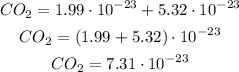
NO₂: 1 atom of nytrogen and 2 atoms of oxygen
We know that:
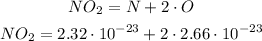
We can see this is really similar to the previous one.
We follow the same procedure:
First we multiply the numbers without exponent: 2 * 2.66:
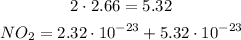
Now, we can add both terms:
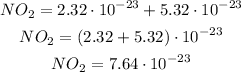
NH₃: 1 atom of nytrogen and 2 atoms of hydrogen
We know that:
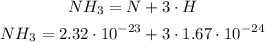
First we multiply the numbers without exponent: 3 * 1.67:
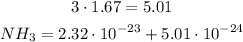
For the first time we have two terms with different 10 power:
on one side 10⁻²³ and on the other 10⁻²⁴
We want both have the same because if they are different we cannot add them.
Then we want to transform
5.01 · 10⁻²⁴
Into
___ · 10⁻²³
We need to find that number
Since
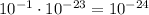
Then
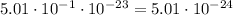
We know that
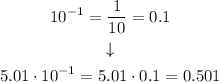
Then
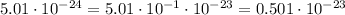
Then, getting back to our equation for NH₃, we have that:
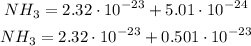
Now, we can add normally as we did on the previous molecules:
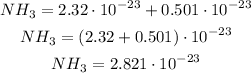
Answer: