Answer
286.356 grams of salt (NaCl) needed to be added.
Step-by-step explanation
Given:
ΔTb = 5 °C
i = 2, because 1 mol of NaCl gives 2 mol of particles in solution.
Kb = 0.51 °Ckg/mol
What to find:
To find how much salt in grams would one need to add to 1 kg of water to change the boiling temperature by 5°C.
Step-by-step solution:
Step 1: Determine the molality of the solution.
The formula for boiling point elevation is:
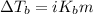
Where

Therefore, the molality of the solution is:
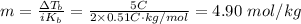
Step 2: Determine the moles of NaCl in 4.90 mol/kg of NaCl.
The formula for molality is:
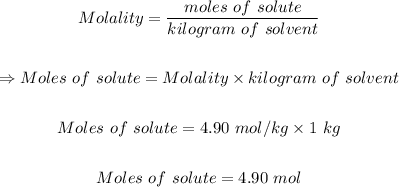
Step 3: Convert moles of NaCl in step 2 to grams of NaCl.
Finally, we can convert the moles of NaCl in step 2 to grams of NaCl using the mole formula below:
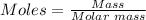
Putting moles of NaCl as 4.90 mol and the molar mass of NaCl as 58.44 g/mol, the mass of NaCl in grams is:
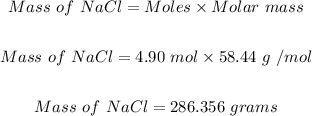
Hence, 286.356 grams of salt (NaCl) is needed to be added to 1 kg of water to change the boiling temperature by 5°C.
.