Rate reaction 2 is faster in 3 significant figure 2.00
Step-by-step explanation:
Reactant of reaction 1 = 0.3mol/L
Reactant of reaction 2 = 0.6mol/L
To determine how many times faster, we find the ration of reactant 2 to reactant 1
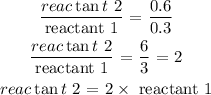
Therefore, reactant 2 is 2 times faster than reactant 1
Rate reaction 2 is faster in 3 significant figures 2.00