Answer:
Explanations:
What is the empirical formula?
The empirical formula of a chemical compound is the simplest whole-number ratio of atoms present in a compound.
The formula that relates the empirical formula to the molecular formula is expressed as:
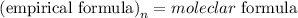
Given the molecular formula of ascorbic acid given as C₆H₈O₆, the empirical formula will be expressed as:
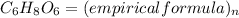
Since the empirical formula of a chemical compound is the simplest whole number, hence dividing the atom through by 2, we will have:
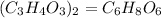
Hence the empirical formula from the given expression will
![undefined]()