To solve this question, we simply need to use the combined gas equation which comprises of Boyle's law, Charles law and pressure law.
This formula is used to find the change of parameter of a gas from either one stage to another or to STP. Basically, it is used to compared or find missing values of gas since their equations are interconnected.
The combined gas equation is given as
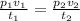
Now, let's defind our given parameters
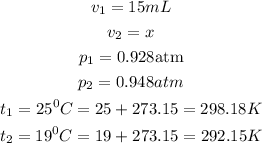
Now, we are seeking for the change in volume of the gas from 15ml to ?
V₂ is equal to
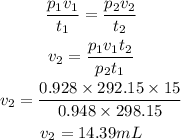
From the calculations above, the change of volume is equal to 14.39mL