So,
According to the reaction:
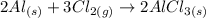
We have an initial amount of 25.0g of Al and excess chlorine.
What we're going to do to find the number of moles of aluminum chloride that could be produced, is to follow the steps:
1. Pass the grams to moles dividing the mass given by the molar mass of Al:
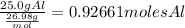
2. State the relation between the coefficients of the reaction. They tell us that each 2 moles of Al produce 2 moles of AlCl3. So,
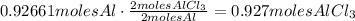
Therefore, 0.927 moles of aluminum chloride could be produced.