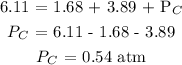Answer:
Step-by-step explanation:
Here, we want to get the partial pressure of gas C
According to Dalton's law of partial pressure, the total pressure of a mixture of gases is equal to the sum of the individual partial pressures
Thus, the total pressure is the sum of the partial pressure of gas A, partial pressure of gas B and partial pressure of gas C
Mathematically, we have that as:
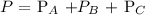
Substituting the values, we have it that: