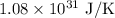Given:
The sun radiates energy at a rate of
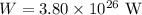
The temperature in the empty space is,
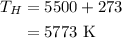
The temperature at the deep space is,
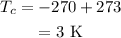
To find:
the increase in entropy (in J/K) in one day
Step-by-step explanation:
The value of heat is,
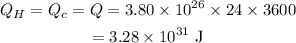
The change in entropy is,
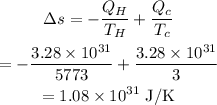
Hence, the increase in entropy is