Answer:
Approximately, 416 g of CO2 will be removed
Step-by-step explanation:
Here, we want to get the mass of LiOH that can be removed
We start by getting the number of moles of LiOH that wants to do the removal
We can get this by dividing the mass by the molar mass of LiOH
The molar mass of LiOH is 24 g/mol
Thus, we have the number of moles performing the removal as:
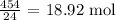
Looking at the balanced equation, 2 moles of LiOH will remove 1 mole of Carbon iv oxide
What this means is that
18.92 moles LiOH will remove 18.92/2 = 9.46 moles
To get the actual mass removed, we multiply this number of moles by the molar mass of CO2
The molar mass of CO2 is 44g/mol
Thus, we have it that the mass removed will be:
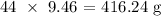
Approximately, 416 g of CO2 will be removed