Consider that the functions are given as,
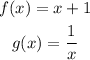
Note that the composition function exists only if both the functions are defined at that point.
Note that the function g(x) is not defined at x=0.
Solve for the composite function as,
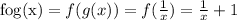
The composite function contains the term 1/x which cannot be zero for any real value of 'x'.
So it follows that the composite function can never take the value 1,
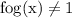
Thus, the range of the composite function should be the set of all possible real numbers except 1.