Step 1 - Understanding mole and molar mass
The molar mass is, by definition, the mass of one mole of something. One mole is a very big number representing 6*10^23 entities.
When we say, for example, that the molar mass of water is 18 g/mol, we mean that one mole of water molecules, i.e., 6*10^23 water molecules, weight 18g.
In order to solve this question, we must first calculate the molar mass of sucrose (C12H22O11). The molar masses for C, H and O are, respectively, 12 g/mol, 1 g/mol and 16 g/mol. Therefore:
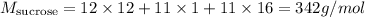
Step 2 - Converting mass of sucrose to moles of sucrose
Now that we have calculated the molar mass of sucrose, we can convert mass to moles:
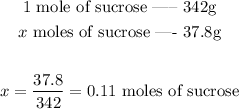
37.8 grams corresponds thus to 0.11 moles of sucrose.