First, we need to start off by finding the angle adjacent (next to) y.
We can find this angle because we know that the angles in a triangle add up to 180 degrees, and we are given 2 of the other angles.
We can find the last angle by subtracting the value of these angles from 180:
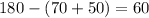
Now, we can find the measure of angle y by using the knowledge that the angle of a straight line is 180 degrees. If y and the angle 60 make up the line, then y must be equal to:
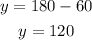
The angle y is equal to 120 degrees.
Now that we have the angle y, we can find angle z by using the same method we did to calculate the missing angle on the lefthand side, next to y.
Let's add up angle y and 28 and subtract this from 180 degrees:
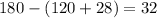
The angle z is equal to 32 degrees.