Answer:
5.66%
Explanations:
The formula for calculating the percent mass/volume of a solution is expressed as:
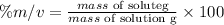
Given the following parameters
Mass of solute = 30000mg = 30g
Mass of solvent = 0.5litres = 500g
Mass of solution = 30g + 500g = 530g
Substitute the parameters into the formula
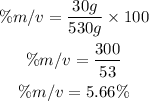
Hence the percent mass/volume of a solution is 5.66%