To solve this problem we must take into account that the balloon will not exert pressure on the gas in case of an expansion, we can also say that there is no gas inlet or outlet. Therefore, we can assume that the moles of the gas and the pressure remain constant.
The law that relates temperature and volume at constant pressure is Charles's law, which is described by the following equation:
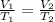
Where,
V1 is the initial volume in liters, 4.25L
T1 is the initial temperature in Kelvin, 45+273.15=318.15K
V2 is the final volume, 8.60L
T2 is the final temperature
We clear T2 and replace the known data
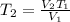
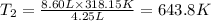
Answer: The ballon will expand at 8.60 L at 643.8K