For this problem, we have to use Avogadro's number which is 6.022 x 10^(23) /mol. This number is telling us that there are 6.022 x 10^(23) atoms in 1 mole of a certain compound/element. The conversion would be:
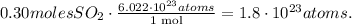
The answer is that there are 1.8 x 10 ^(23) atoms of SO2 in 0.30 moles.