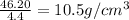Answer:
Explanation;
Here, we want to get the density of silver
We start by getting the mass of the silver
That would be the difference between the total mass after insertion of silver and before
We have this as:'
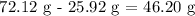
Now, we want to get the volume of the silver which is equal to the volume of water displaced
Mathematically, we have that as:
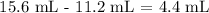
Mathematically, we know that mL is the same as cm^3, so the value of the volume remains the same
Finally, we can get the density by dividing the mass of the silver by the volume of water displaced
Mathematically, we have that as: