Answer:
The temperature is 7.94°C.
Step-by-step explanation:
1st) To calculate the temperature, we can use the Charles's Law.
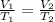
The given information is:
- V1: 8.5L
- T1: 15.0°C
- V2: 4.5L
- T2: this is what we have to calculate:
2nd) Replacing the values in the formula:
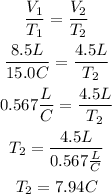
Finally, the temperature is 7.94°C.