Answer
1917149.087 grams of (NH₄)₂SO₄ are in 87.37 x 10²⁶ atoms of (NH₄)₂SO₄.
Step-by-step explanation
Given that:
The number of atoms of (NH₄)₂SO₄ = 87.37 x 10²⁶ atoms
What to find:
To find the grams of (NH₄)₂SO₄ in 87.37 x 10²⁶ atoms of (NH₄)₂SO₄.
Step-by-step solution:
Step 1: Convert 87.37 x 10²⁶ atoms of(NH₄)₂SO₄ to moles.
Conversion factor: One mole of a substance will contain 6.022 × 10²³ atoms of that substance
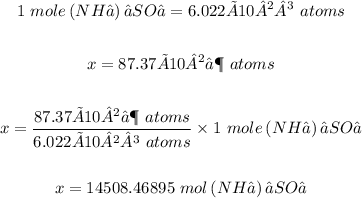
Step 2: Convert 14508.46895 moles of (NH₄)₂SO₄ to grams.
Using the molar mass of (NH₄)₂SO₄ = 132.14 g/mol and the mole formula below, the grams of (NH₄)₂SO₄ can be determined as follows:
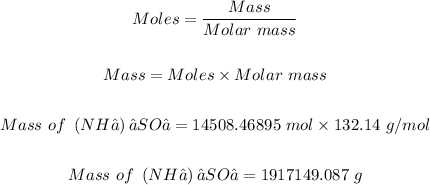
Therefore, 1917149.087 grams of (NH₄)₂SO₄ are in 87.37 x 10²⁶ atoms of (NH₄)₂SO₄.