In the most used STP conditions, the volume occupied by 1 mol of a gas is 22.4 L.
That is, 1 mol to 22.4 L. Since we know we have 22.2 mol of a gas in STP, we can use the rule of three to find its volume:
1 mol --- 22.4 L
22.2 mol --- V
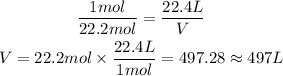
So, the volume is approximately 497 L.