Data:
• Product expectation: 7.40 umol
,
• Actual production: 75% of the expected.
Procedure
First, we have to get the proportion of 75 ad follows:
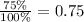
Then, we have to multiply that proportion to the product expectation:
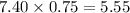
5.55 umol of carbon dioxide were produced. However, the problem is asking in mol, thus we have to make the following conversion:
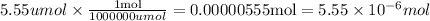
Answer: 0.00000555