Step-by-step explanation
Given
Mass of BaO produced = 5.75g
Required: Heat released when BaO is produced
Solution
Step 1: Calculate the energy released per mole of BaO
BaO has a coefficient of 2, meanibg there are 2 moles.
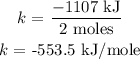
Step 2: Calculate the number of moles of BaO
n = m/M where n is the moles, m is the mass and M is the molar mass
n = 5.75g/153.33 g/mol
n = 0.0375 mol
Step 3: Calculate the energy released
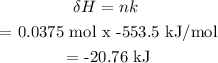
Answer
Energy released = 20.8 kJ