Given
m = 1g
Lvap = 2260 j/g
p = 1.01 x 10^5 Pa = 1 Atm
T = 100 C = 373 K
Vsteam = 1671 cm3
Vwater = 1 cm3
ΔV = (Vsteam - Vwater) = 1671 - 1 = 1670 cm3
Procedure
a) External work done by the system
Q = mL
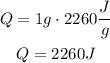
W = pΔV
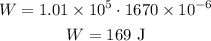
External word done by the system is 169 J
b) increase in internal energy
ΔU = Q - W
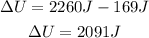
Increase in the internal energy is 2091 J