Step 1 - Relating concentration, volume and number of moles
The definition of molar concentration (M) is the quotient between the number of moles (n) and the total volume of the solution (V). We can state it in a mathetical way as:
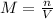
We can now rearrange this equation to obtain the number of moles, n:
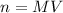
We'll use this relation to solve this exercise.
Step 2 - Finding the number of moles of NaOH
The concentration of the solution is 2.2 M and its volume is 0.065 L (65 ml). Therefore:
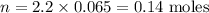
There are thus 0.14 moles of NaOH in 65 ml of this solution.