Answer:
We first look up in the periodic table the molar mass of each individual element:
Fe: 55.84 g/mol
Cl: 35.45 g/mol
Then we calculate the molar mass of the compound:
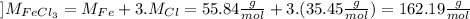
Finaly we calculate the percentage:
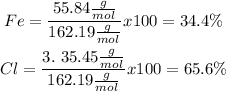
So finally, the percentages of each element in the molecule are:
Fe: 34.4%
Cl: 65.6%