A neutral solution has a pH equal to 7. We can apply the definition of pH to find the concentration of H3O+ ions. The equation that describes pH is:
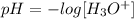
If we clear the concentration of H3O+ ions we have:
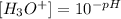
Now, we replace the pH equal to 7:
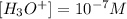
The concentration of ions H3O+ in a neutral solution is 10^-7M. so the statement is false.
Answer: False