STEP - BY - STEP EXPLANATION
What to find?
Average rate of the reaction from 20-40 seconds.
Given:
The average rate of the reaction from 20-40 seconds can be calculated following the steps below:
Step 1
State the formula.
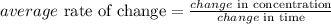
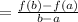
From the graph given:
If a =20, f(a) =0.14
If b =40 , f(b) = 0.11
Step 2
Substitute the values into the formula.
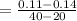
Step 3
Simplify
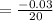
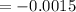
ANSWER
The average rate of change is -0.0015 M/L/sec.