Answer:
True.
Step-by-step explanation:
The pH scale measures the concentration of hydrogen ions in acidic and alkaline solutions.
In chemistry, it literally means power of hydrogen ions and it is a measure of the molar concentration of hydrogen ions in a particular solution, thus specifying the acidity, neutrality or basicity of chemical solutions.
Mathematically, the pH of a solution is given by;
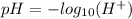
Hence, a solution with a pH of 7 is neutral. Also, a solution with a pH below 7 is acidic but basic (alkaline) if it's pH is above 7.