Answer:
3.0 moles of aluminum ions
Explanations:
An Aluminium oxide Al₂O₃ consists of;
• two ,aluminum cations 2Al³⁺ and;
,
• three ,oxygen anions 3O²⁻
Based on stoichiometry, one mole of aluminum oxide therefore contains;
• two ,aluminum cations 2Al³⁺ and;
,
• three ,oxygen anions 3O²⁻
Given the parameter:
Number of moles of aluminum oxide = 1.5 moles
The number of moles of aluminum ion is calculated as thus:
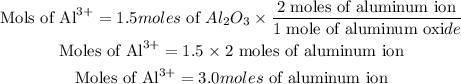
Hence the number of moles of aluminum ions in 1.5 moles of Al₂O₃ is 3.0 moles