The wavelengths which are absorbed by the hydrogen atom are all multiples of the fundamental radius, which is a0=0.0529nm. A wavelength of 103nm corresponds to:
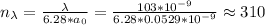
So the next will be 311, which correspondes to a wavelength of:
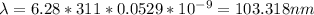
Thus, our answer is lambda=103.318nm