The balanced equation of reaction they describe is the following:
NaOH + KHP → KNaP + H2O
By stoichiometry, we have that the ratio NaOH and KHP is one to one, which means that if we have n moles of KHP we will need the same n moles NaOH.
Now, let's determine the moles of KHP using its molecular mass:

Therefore, we must add 1.577x10^-3 moles of NaOH. The molarity is defined as:
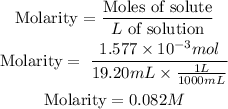
The molarity of NaOH solution is 0.082M