We are given the balanced equation for the reaction, so we can continue with the calculations.
To find the grams of NO2 produced, we will follow these steps:
1. We find the moles of HNO3 present in 35 grams. For that we divide the grams by the molar mass of HNO3. The molar mass of HNO3 is: 63.01g/mol
2. We find the theoretical moles of NO2 that are formed. By stoichiometry, the ratio NO2 to HNO3 is 3/2.
3. We find the theoretical grams of NO2 that are formed by multiplying the moles found by the molar mass of NO2.
4. We find the grams produced using the yield term which is described by the following equation:

Let's proceed with the calculations.
1. Moles of HNO3
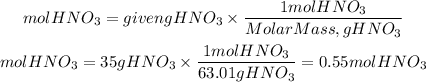
2. Theoretical Moles of NO2
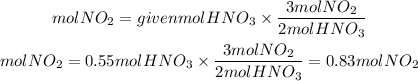
3. Theoretical Grams of NO2
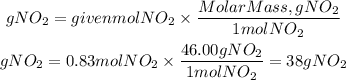
4. Actual grams of NO2
![\begin{gathered} ActualY\imaginaryI eld=\frac{45.9\%*38gNO_2}{100\operatorname{\%}} \\ ActualY\mathrm{i}eld=17.6gNO_2 \end{gathered}]()
Actual Yield= (45.9% x 38 g NO2)/100%
Actual Yield= 17.6 g NO2
Answer: It will be produced 17.6 grams of NO2