We know
D = RT
Where
D is distance
R is rate
T is time
It is given that Brand A scooter (the faster one) traveled 75 miles in 5 hours.
Given
D = 75
T = 5
Let's find R:
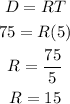
Brand A scooter's Rate = 15 mph
We know Brand A is 4 mph FASTER than Brand B. So,
Brand B is 4 mph slower than 15, hence Brand B's speed (rate) would be:
15 - 4 = 11 mph
From the answer choices,
the correct answer is D