Answer
V2 = 10.95 L
Step-by-step explanation
Given:
mass 1 = 4.20 g
mass 2 = 2.25 g
volume 1 = 20.5 L
Required: volume 2
Solution
Step 1: Find the number of moles of mass 1 and 2.
For mass 1:
n = m/M where n is the moles, m is the mass and M is the molar mass
n = 4.20g/31.998g/mol
n = 0.131 mol
For mass 2:
n = 2.25g/31.998g/mol
n = 0.070 mol
Step 2: For this problem, we will use the ideal gas law equation
PV = nRT
However, P, V and R are constant
Therefore
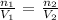
V2 = (V1n2)/n1
V2 = (20.5L x 0.070 mol)/0.131 mol
V2 = 10.95 L