Answer
B) 16.7
The final temperature of this gas in degrees Celsius = 16.7 °C
Step-by-step explanation
Given:
Initial volume, V₁ = 214.0 mL
Initial pressure, P₁ = 616.20 mmHg
Initial temperature, T₁ = 11.9 °C
Final pressure, P₂ = 261.90 mmHg
Final volume, V₂ = 512.0 mL
What to find:
The final temperature of this gas in degrees Celsius.
Step-by-step solution:
The first step is to convert the temperature to K.
Conversion factor:
0 °C = 0 + 273.15 K
∴ T₁ = 11.9 °C = 11.9 + 273 K = 285.05 K
The next step is to calculate the final temperature in K using the combined gas equation.
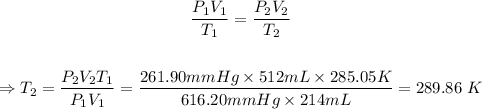
The final step is to convert 289.86 K to °C.
Conversion factor:
0 K - 273.15 = -273.15 °C
Therefore, 289.86 - 273.15 = 16.71 °C = 16.7 °C
The final temperature of this gas in degrees Celsius is 16.7 °C