Answer:
There are 0.26 moles of neon.
Step-by-step explanation:
From the information in the exercise we know that:
- Volume (V): 2.00L
- Temperature (T): 300.0K
- Pressure (P): 3.25atm
Using the Ideal Gases Law formula, we can calculate the number of moles (n), by replacing the values of V, T and P:
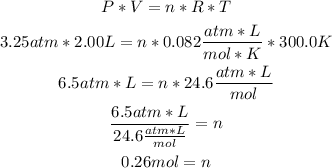
So, there are 0.26 moles of neon (0.3 rounded).