Mass of Oxygen: 0.0159 grams
Moles of Oxygen: 9.94x10^-4
To find the mass of oxygen, subtract the mass of copper from the total mass.
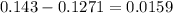
There are 0.0159 grams of Oxygen.
To find how many moles there are, divide the given amount of oxygen by the molar mass (atomic mass) of oxygen because that mass is the same as one mole of oxygen.
Molar mass of Oxygen: 16.00
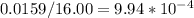
There are 9.94*10^-4 moles of Oxygen.