Given:
λ = 940 μm
Let's solve for the following:
• (a). What is the frequency of this radiation?
To find the frequency, apply the formula:
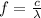
Where:
f is the frequency
c is the speed of light = 3 x 10⁸ m/s
λ = 940 μm
Thus, we have:
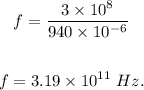
Therefore, the frequency of this radiation is 3.19 x 10¹¹ Hz.
• (b). What type of electromagnetic waves are these?
Since the wave has a frequency of 3.19 x 10¹¹ Hz, the wave will be said to be an infrared.
• (c). How much energy (in electron volts) is carried by one quantum of this radiation?
Apply the formula:
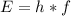
Where:
E is the energy in Joules
h is Planck's constant = 6.626 x 10⁻³⁴
f is the frequency gotten in part A.
We have:
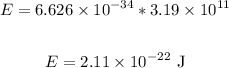
Now, to convert from Joules (J) to electron volts (eV), we have:
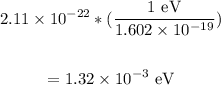
Therefore, the energy in electron volts is 1.32 x 10⁻³ eV.
ANSWER:
• (a). 3.19 x 10¹¹ Hz.
• (b). Infrared
• (c). 1.32 x 10⁻³ eV.