The total number of moles is something relative. If we wanted to be critical, we would have to consider the dissociation of the ions, if in solution, and the number of moles of solvent (if there is one).
However, the hint says that to calculate the number of moles we would have to divide each mass by the corresponding molar mass and add them.
This means that we want the total number of moles of each compound that was added to the mixture, so it is probably just a mixture of the solids.
So, we want the following:
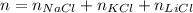
The molar mass, M, is the property that relates the number of moles, n, and the mass, m, of a compound:
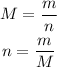
The molar masses are given in the question, so we don't need to calculate them:
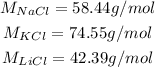
So, we have:
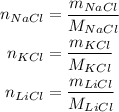
So, putting them into the equation, we have:
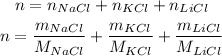
Now, we just need to substitute the given values:
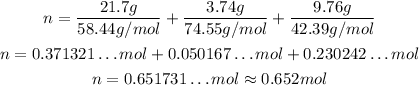
So, there are approximately 0.652 mol in the mixture.