Answer: The heat of combustion per gram of the material is 53.5 kJ
Step-by-step explanation:
Let the heat released during reaction be q.
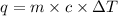
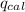 = Heat gained by calorimeter
= Heat gained by calorimeter
Heat capacity of bomb calorimeter ,C = 38.29 kJ/°C
Change in temperature = ΔT = (27.04-23.61) °C = 3.43 °C
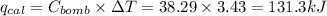
Total heat released during reaction is equal to total heat gained by bomb calorimeter.
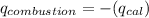
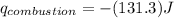
Thus 2.455 g of material releases 131.3 kJ of heat
1 g of material releases =
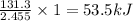 of heat
of heat
Thus the heat of combustion per gram of the material is 53.5 kJ