ANSWER
The number of the unpaired electrons is 1
Explanation:
The electronic configuration of a particular element is given below as
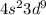
From the electronic configuration above, you will see that the 4s-orbital has two electrons and the 3d-orbital has 9 electrons
According to Hund's Rule, it "states that electron goes into orbital singly before pairing commences."
To determine the number of unpaired electrons, we need to study the total number of electrons in the d-orbital as given by the question.
Note that, the maximum number of electrons that can enter into the d-orbital is 10
The next step is to fill in the electrons into the orbital
From the above structure, you will see that the number of paired electrons is 4 and the number of unpaired electrons is 1
Therefore, the number of the unpaired electrons is 1