Answer:
The color of the new solution is due to that a redox reaction took place, in which the copper was oxidized from Cu(0) to Cu(+2), in the copper nitrate solution.
Step-by-step explanation:
Silver nitrate (AgNO3) reacts with copper (Cu) like we can see in the following reaction:
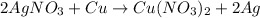
Silver crystals are formed by reduction of silver, while copper is oxidized, forming copper nitrate, resulting in a blue colored solution.
The color of the new solution is due to that a redox reaction took place, in which the copper was oxidized from Cu(0) to Cu(+2).
Copper with oxidation number of 0, in the solid state, has a coppery color. When copper is oxidized to copper with +2 oxidation number (to form part of copper nitrate, Cu(NO3)2) in solution, it turns blue when in contact with water molecules that surround the copper nitrate molecules.