1) List the known and unknown quantities.
Final conditions
Volume 2: 10.0 L
Concentration 2: 1.2 M
Final conditions
Volume 1: 2.5 L
Concentration 1: unknown
2) Set the equation
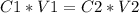
3) Plug in the known values and solve for C1
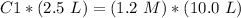
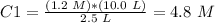
The molarity would be 4.8 M.
.