We want to determine the equation that best model the given data.
we will take each of the equation and substitute the value of t into the functions to determine the best fit. That means the one with the closest value to the given data;
Firstly, for the first option;
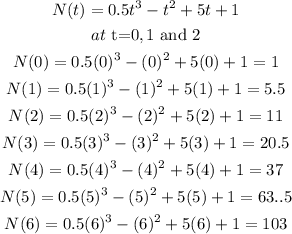
Then the second option;
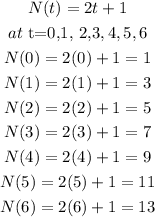
the Third option;
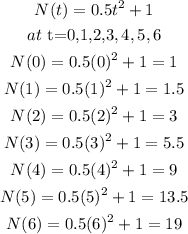
And lastly;
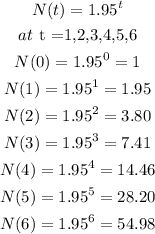
So, from the given results the best fit function to model the equation is the last option because the results are approximately equal to the one presented in the table.
Therefore, the function that best models the given data is:
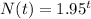
Note;
In a case when you need to be fast to can substitute just three of the values of t and compare. Like at t=0,3 and 6.
Attached is a graph of each of the model and the data given.