To solve this question, we would use the combined gas equation which comprises of Boyle's law, Charles law and pressure law.
The equation is given as
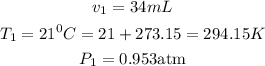
At STP (standard temperature and pressure), the temperature and pressure changes to
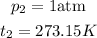
Now, combining the data above, we can find the change in volume (v₂)
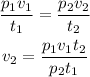
Let's substitute the values into the equation above and solve for v₂
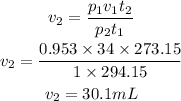
From the calculations above, the volume at STP is 30.1mL